Answer:-
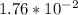 g of carbon dioxide.
g of carbon dioxide.
Solution:- The balanced equation for the combustion of methane is:
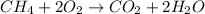
There is 1:1 mol ratio between methane and carbon dioxide. Grams of methane are converted to moles and then using mol ratio we get the moles of carbon dioxide that could further be converted to grams. The calculations are shown as:
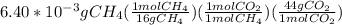
=
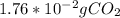
So, complete combustion of given amount of methane gives
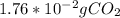 .
.