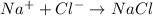Answer: 1) C. Strong attraction between ions.
2) A. High melting points
Step-by-step explanation: Ionic compounds are formed by combination of positively charged ions called cations formed by less electronegative metals and negatively charged ions called anions formed by more electronegative nonmetals.
The cations and anions with opposite charges are bonded through strong coloumbic forces and thus are difficult to break and have high melting points and thus exist as solids at room temperature.
![Na:11:[Ne]3s^1](https://img.qammunity.org/2019/formulas/chemistry/middle-school/9mmkbcsmd3paf9b6gy65l23j9f55a122a9.png)
![Na^+:10:[Ne]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/7qssinjf8a9mk6za6k1qh2xqsetu75zkbq.png)
![Cl:17:[Ne]3s^23p^5](https://img.qammunity.org/2019/formulas/chemistry/high-school/zy893j53wmcgbq8i5ddsar4g9uljy9z9jn.png)
![Cl^-:18:[Ne]3s^23p^6](https://img.qammunity.org/2019/formulas/chemistry/high-school/redxr6xi71t81wqdzzf7ydq90ydsgvyrhe.png)