Answer:- C. Hafnium.
Solution:- Mass of the sample is 46.0 g and it's volume is
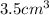 .
.
From mass and volume, we can calculate it's density using the formula:
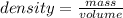
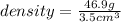
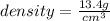
On the basis of the density, this substance could either be mercury or hafnium. Since the substance is a solid at room temperature where as mercury is liquid. So, it can't be mercury.
The right choice is C) Hafnium.