Answer : 0.0392 grams of Zn metal would be required to completely reduced the vanadium.
Explanation :
Let us rewrite the given equations again.
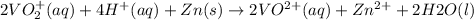
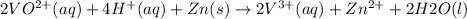
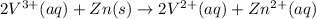
On adding above equations, we get the following combined equation.
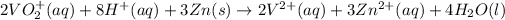
We have 12.1 mL of 0.033 M solution of VO₂⁺.
Let us find the moles of VO₂⁺ from this information.
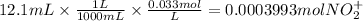
From the combined equation, we can see that the mole ratio of VO₂⁺ to Zn is 2:3.
Let us use this as a conversion factor to find the moles of Zn.
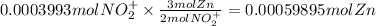
Let us convert the moles of Zn to grams of Zn using molar mass of Zn.
Molar mass of Zn is 65.38 g/mol.
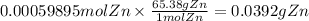
We need 0.0392 grams of Zn metal to completely reduce vanadium.