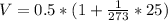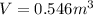To answer this question, we must use the equation for the volumetric expansion of gases at constant pressure. This equation is given by:

We know:
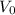 is the initial volume
is the initial volume
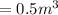
ΔT is the temperature change = 45 ° -20 ° = 25 °
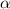 is the coefficient of gas expansion and is equal to 1/273
is the coefficient of gas expansion and is equal to 1/273
Then the final volume of the gas is: