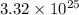Answer : The number of atoms of hydrogen are
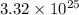
Explanation : Given,
Moles of ammonium sulfide = 6.90 moles
The chemical formula of ammonium sulfide is,
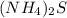
In ammonium sulfide, there are 2 atoms of nitrogen, 8 atoms of hydrogen and 1 atom of sulfur.
As we know that,
1 mole of substance always contains
 number of atoms.
number of atoms.
As, 1 mole of ammonium sulfide contains
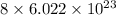 number of hydrogen atoms
number of hydrogen atoms
So, 6.90 mole of ammonium sulfide contains
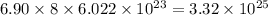 number of hydrogen atoms.
number of hydrogen atoms.
Therefore, the number of atoms of hydrogen are