Answer:
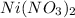
Step-by-step explanation: A molecule is made up by combination of same atoms or different atoms.
1 molecule of
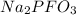 consists of 2 atoms of sodium, 1 atom of phosphorous, 1 atom of flourine and three atoms of oxygen. Thus the total number of atoms is 7.
consists of 2 atoms of sodium, 1 atom of phosphorous, 1 atom of flourine and three atoms of oxygen. Thus the total number of atoms is 7.
1 molecule of
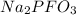 consists of 2 atoms of sodium, 1 atom of phosphorous, 1 atom of flourine and three atoms of oxygen. Thus the total number of atoms is 7.
consists of 2 atoms of sodium, 1 atom of phosphorous, 1 atom of flourine and three atoms of oxygen. Thus the total number of atoms is 7.
1 molecule of
 consists of 2 atoms of hydrogen, 1 atom of sulfur and 4 atoms of oxygen. Thus the total number of atoms is 7.
consists of 2 atoms of hydrogen, 1 atom of sulfur and 4 atoms of oxygen. Thus the total number of atoms is 7.
1 molecule of
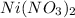 consists of 1 atom of nickel, 2 atoms of nitrogen and 6 atoms of oxygen. Thus the total number of atoms is 9.
consists of 1 atom of nickel, 2 atoms of nitrogen and 6 atoms of oxygen. Thus the total number of atoms is 9.
1 molecule of
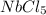 consists of 1 atom of niobium and 5 atoms of chlorine. Thus the total number of atoms is 6.
consists of 1 atom of niobium and 5 atoms of chlorine. Thus the total number of atoms is 6.
Thus substance which contains the most total atoms per molecule is
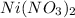 .
.