Answer: Option (B) is the correct answer.
Step-by-step explanation:
An exothermic reaction is defined as the chemical reaction in which there occurs release of heat.
For example,
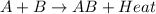
In an exothermic reaction, energy of reactants is more than the energy of products.
Whereas an endothermic reaction is defined as a chemical reaction in which heat is absorbed by reactant molecules.
For example,
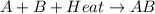
In endothermic reaction, energy of reactant molecules is less than the energy of products.
Thus, we can conclude that release of heat and/or light energy indicates that an exothermic reaction has taken place.