Answer: a) 1.97 grams of carbon disulfide will remain after 37.0 days.
b) 2.85 grams of carbon monosulfide will be formed after 37.0 days.
Step-by-step explanation: The decomposition of carbon disulfide is given as:
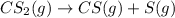
at t=0 4.83g 0 0
at t=37 days 4.83 - x x x
here,
x = amount of
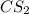 utilised in the reaction
utilised in the reaction
This reaction follows first order kinetics so the rate law equation is:
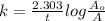
where, k = rate constant
t = time
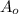 = Initial mass of reactant
= Initial mass of reactant
A = Final mass of reactant
a) For this, the value of
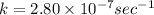
t = 370 days = 3196800 sec
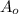 = 4.83
= 4.83
A = 4.83-x
Putting values in the above equation, we get
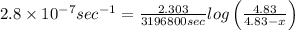
x = 2.85g
Amount of
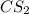 remained after 37 days = 4.83 - x
remained after 37 days = 4.83 - x
= 1.97g
b) Amount of carbon monosulfide formed will be equal to "x" only which we have calculated in the previous part.
Amount of carbon monosulfide formed = 2.85g