Answer:- 6200 kg
Solution:- Volume of bromine is given as
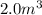 and it's density is
and it's density is
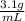 . It asks to calculate the mass of bromine in kg.
. It asks to calculate the mass of bromine in kg.
We need to do the unit conversions for volume from
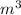 to
to
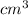 and then mL. After this the volume is multiplied by density to get the mass in grams that could further be converted to kg. The set is shown as:
and then mL. After this the volume is multiplied by density to get the mass in grams that could further be converted to kg. The set is shown as:
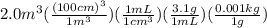
= 6200 kg
So, the mass of bromine in the given volume of it is 6200 kg.