Heat required to raise the temperature of a given system is
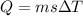
here we know that
m = mass
s = specific heat capacity
 = change in temperature
= change in temperature
now as we know that
mass of wood = 5 kg
mass of aluminium pan = 2 kg
change in temperature = 45 - 20 = 25 degree C
specific heat capacity of wood = 1700 J/kg C
specific heat capacity of aluminium = 900 J/kg C
now here we will find the total heat to raise the temperature of both
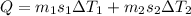
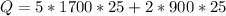
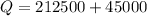
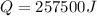
So heat required to raise the temperature of the system is 257500 J