Answer: The average atomic mass of this elements is 56.7221 amu.
Explanation: The average atomic mass is the sum of the masses of its isotopes each multiplied by their natural abundances.
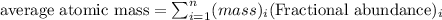 .....(1)
.....(1)
We are given 3 isotopes of an element.
For Isotope
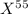 ,
,
Mass = 55 amu
Fractional abundance = 0.2780
For isotope
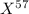 ,
,
Mass = 57 amu
Fractional abundance = 0.4439
Total Fractional abundance = 1
For isotope
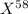 ,
,
Mass = 58 amu
Fractional abundance = Total abundance - abundances of the other isotopes
Fractional abundance = 1 - 0.7219
= 0.2781
Now, putting all the values in equation 1, we get
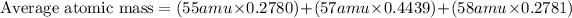
Average atomic mass = 56.7221 amu.