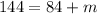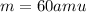Answer:
m = 60 amu
Step-by-step explanation:
As we know that total mass is always conserved
So here we know that compound is of mass 84 amu initially
after reaction with vinegar molecule the total mass is given as

here we know that
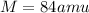
so we will have
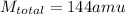
so we have