Answer: Option 1, 4 and 5 describe acids accurately.
Step-by-step explanation:
Option 1: Acids have a sour taste because of the concentration of
 ion.
ion.
Option 2: They are corrosive in nature, that is they are not at all gentle with the skin and fabric as some acids intend to burn skin such as
 is a strong acid which can burn skin as well as fabric.
is a strong acid which can burn skin as well as fabric.
Option 3: Acids need electrons to release
 ions and non-metals do not donate electrons hence, there is no reaction between a non-metal and an acid.
ions and non-metals do not donate electrons hence, there is no reaction between a non-metal and an acid.
Option 4: All acids do contain hydrogen. They dissociate in the presence of water to produce
 ions.
ions.
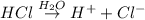
Option 5: Acids do conduct electricity that is they carry electrical charges. This was explained by Arrhenius. He said that the acids dissociate into
 ions, when it is dissolved in water. These ions hence acts as charge carriers in water.
ions, when it is dissolved in water. These ions hence acts as charge carriers in water.