Answer:
An ideal gas is a theoretical concept and a real gas behaves in an ideal manner under conditions which includes a high temperature and a low pressure such that the gas has high kinetic energy, and the intermolecular forces between the molecules are weak
The Ideal Gas Law is P·V = n·R·T
Where;
P = The pressure of the gas
V = The volume of the gas
n = The number of moles of the gas
R = The Universal Gas Constant
T = The temperature of the gas
For a gas cooled to 10 Kelvin and placed under high pressure, the interaction between individual gas molecules increases, and the kinetic energy of the gases is much lower and more comparable to the inter molecular forces between the gas molecules, which in turn produces observable changes from the initial ideal behavior of the gas such that the gas behavior deviates from the Ideal Gas Laws appreciably and are better modelled by the Van der Waals Equations which takes into account, the volume the gas particles occupy and the intermolecular forces between the molecules
The Van der Waals equation is presented as follows;
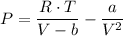
Where;
V = The molar volume
a = The gas constant a represents the attractive forces between the gas particles
b = Represent the volume occupied by the particles of the gas
Step-by-step explanation: