Answer:- 8.35 kJ
Solution:- It asks to calculate the heat required to melt 25 g of ice at 0 degree C. It is a phase change and the heat energy is calculated by using the formula:
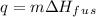
where q is the heat energy, m is the mass and
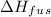 is the enthalpy of fusion of ice. Enthalpy of fusion of ice is 334 Joule per gram.
is the enthalpy of fusion of ice. Enthalpy of fusion of ice is 334 Joule per gram.
Let's plug in the given values in the formula:
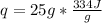
q = 8350 J
since, 1000 = 1 kJ
So,

= 8.35 kJ
So, 8.35 kJ of heat is required to melt 25 g of ice at 0 degree C.