Molarity is defined as the ratio of number of moles to the volume of solution in litres.
The mathematical expression is given as:
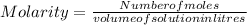
Here, molarity is equal to 1.43 M and volume is equal to 785 mL.
Convert mL into L
As, 1 mL = 0.001 L
Thus, volume =
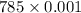 = 0.785 L
= 0.785 L
Rearrange the formula of molarity in terms of number of moles:
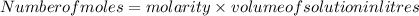
n =

= 1.12255 mole
Now, Number of moles =
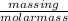
Molar mass of potassium hydroxide = 56.10 g/mol
1.12255 mole =
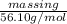
mass in g =
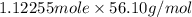
= 62.97 g
Hence, mass of
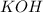 = 62.97 g
= 62.97 g