Answer: The amount of heat required for melting of ice is 4277 J
Step-by-step explanation:
The chemical equation for the phase change of ice follows:
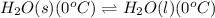
To calculate the amount of heat required to melt the ice at its melting point, we use the equation:
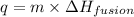
where,
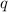 = amount of heat absorbed = ?
= amount of heat absorbed = ?
m = mass of ice = 12.8 g
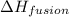 = enthalpy change for fusion = 334.16 J/g
= enthalpy change for fusion = 334.16 J/g
Putting all the values in above equation, we get:
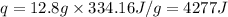
Hence, the amount of heat required for melting of ice is 4277 J