Answer
The answer of given question is 0.13 mol
Step-by-step explanation
1 mol of chlorine (Cl) contain =
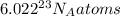 atoms (Avogadro Number)
atoms (Avogadro Number)
So, we have to determine how many moles of Cl will be contain for
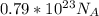 atoms.
atoms.
As we know unit method
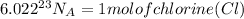
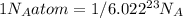 }
}
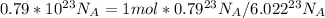
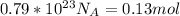
So, the answer is 0.13 mol