Step-by-step explanation:
From the atomic emission spectrum of Mercury, the wavelength of the dark green line is 545 nm.
The relation between the frequency and wavelength is given by :
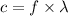
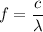
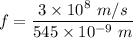
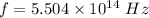
Let E is the energy of an dark green photon emitted by the mercury atom. It is given by :
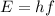
h is Planck's constant
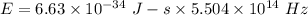
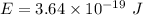
Hence, this is the required solution.