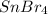Answer : The correct option is, tin(IV) bromide,
Explanation :
Ionic compound : It is defined as the compound which is formed when electron gets transferred from one atom to another atom.
Ionic compound are usually formed when a metal reacts with a non-metal.
The nomenclature of ionic compounds is given by:
1. Positive ion is written first.
2. The negative ion is written next and a suffix is added at the end of the negative ion. The suffix written is '-ide'.
3. In case of transition metals, the oxidation state are written in roman numerals in bracket in-front of positive ions.
(a) tin(IV) bromide
Tin(IV) bromide is an ionic compound because tin element is a metal and bromide element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
The charge on tin is (+4) and the the charge on bromide is (-1). Thus, the formula of the compound tin(IV) bromide will be
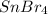
(b) potassium chloride
Potassium chloride is an ionic compound because potassium element is a metal and chloride element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
The charge on potassium is (+1) and the the charge on chloride is (-1). Thus, the formula of the compound potassium chloride will be
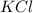
(c) iron(II) oxide
Iron(II) oxide is an ionic compound because iron element is a metal and oxide element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
The charge on iron is (+2) and the the charge on oxide is (-2). Thus, the formula of the compound iron(II) oxide will be
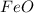
(d) aluminum fluorate
Aluminum fluorate is an ionic compound because aluminium element is a metal and fluorate element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
The charge on aluminium is (+3) and the the charge on fluorate is (-1). Thus, the formula of the compound aluminum fluorate will be
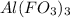
Hence, the correct option is, tin(IV) bromide,