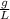Answer:
The mass concentration of fat is 16.96
Step-by-step explanation:
The ratio between the mass of the solute and the mass of the resulting mixture is defined as percentage mass concentration (% m / m). This is:
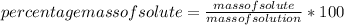
Then, in this cases the percent of mass in fat is:
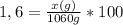
Isolating the value of x is obtained:
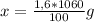
x= 16.96 g
Considering that:
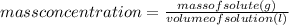
Then:
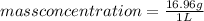
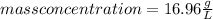
So, the mass concentration of fat is 16.96