Step-by-step explanation:
As number of moles is equal to mass divided by molar mass. As molar mass of calcium cyanide is 92.11 g/mol.
So, calculate the mass of calcium cyanide as follows.
No. moles of calcium cyanide =
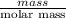
0.69 mol =
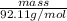
mass = 63.55 g
Thus, we can conclude that mass of calcium cyanide is 63.55 g.