Here we have to get the symbol of the selenium ion having two extra electrons.
The symbol of the selenium ion with two extra electrons is
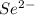 .
.
The number of electrons present in selenium is 34. The electronic configuration of selenium (Se) is 1s²2s²2p⁶3s²3p⁶3d¹⁰4s²4p⁴.
Now we can see that there are 4 electrons in p-orbital. The p-orbital can take maximum 6 electrons thus there is two vacant space in 4p orbital for selenium. If we add two electrons then the total number of electrons will be 36. The electronic configuration can be written as:
[Se²⁻] = 1s²2s²2p⁶3s²3p⁶3d¹⁰4s²4p⁶.
The two negative charge on selenium makes it an anion as one electron always posses one unit negative charge. Thus for the incorporation of the two electrons there will be accumulation of two negative charge on the selenium atom and it will be expressed as Se²⁻.