Answer:
Step 1: slow dissociation of fluorine (controlling stage):
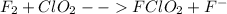
Step 2: fast chlorine dioxide reaction with fluoride ions:
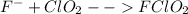
Step-by-step explanation:
Hello,
The undergoing chemical reaction:

Could be attained via two steps forming the required plausible mechanism with the given reaction rate:
Step 1: slow dissociation of fluorine (controlling stage):
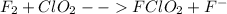
Step 2: fast chlorine dioxide reaction with fluoride ions:
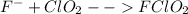
In this case, it is necessary for the fluorine to be dissociated to promote the formation of the two
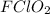 molecules and its consequent simplification as the formed fluorine ions act as intermediates, thus, the overall reaction turn out into:
molecules and its consequent simplification as the formed fluorine ions act as intermediates, thus, the overall reaction turn out into:

Best regards.