Answer:- oxygen.
Explanations:- The electronic configuration is given and we are asked to figure out the electrically neutral atom that will have the electron configuration,
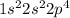 .
.
The sum of electrons for this electron configuration is 8. If we look at the periodic table then 8 is the atomic number of oxygen.
So, the electrically neutral atom for the given electron configuration is oxygen.