The properties like shape, structure, etc. that define the appearance of matter is said to be its physical properties whereas the properties like oxidation, toxicity, etc. that defines how a new product is formed from the other substances is said to be its chemical properties.
It is given that a 2.0 L volume of hydrogen gas is combined with 1.0 L of oxygen gas to produce 2.0 L of water vapor. The chemical reaction is as:
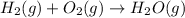
Since, the reaction between hydrogen and oxygen results in the formation of complete new product, water. So, oxygen will undergo a chemical change.