Step-by-step explanation:
A covalent bond is defined as the bond formed due to sharing of electrons between the two chemically combining atoms.
For example,
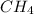 is a covalent compound as sharing of electrons take place between carbon and hydrogen atoms.
is a covalent compound as sharing of electrons take place between carbon and hydrogen atoms.
On the other hand, an ionic bond is defined as the bond formed due to transfer of electrons from one atom to another.
For example, LiCl is an ionic compound as an electron is transferred from Li atom to Cl atom.
A hydrogen bond is defined as a weak bond that is formed between an electropositive atom (generally hydrogen atom) and an electronegative atom like oxygen or nitrogen.
Due to difference in the electronegativity of atoms in a compound containing hydrogen bond there occurs a partial positive charge on hydrogen atom and a partial negative charge on the electronegative atom.
For example,
 is a polar molecule and contains hydrogen bonds.
is a polar molecule and contains hydrogen bonds.
Thus, we can conclude that given bonds are matched as follows.
A. Hydrogen bond
- In this type of bond a molecule has a slightly negative side and a slightly positive side.
- The type of bond that holds two water molecules together.
- The type of bond that hold the hydrogen and oxygen together in a water molecule.
B. Covalent bond
- Atoms in this type of bond share electrons.
C. Ionic bond
- In this type of bond atoms gain or lose electrons to form ions.