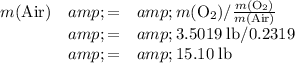Approximately
 pounds.
pounds.
Start by balancing the equation for the complete combustion of octane in oxygen:
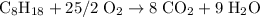
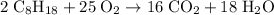
Thus it would take
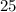 molecules of oxygen to completely react with
molecules of oxygen to completely react with
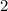 molecules of octane. Oxygen and octane thus react at a ratio of
molecules of octane. Oxygen and octane thus react at a ratio of
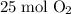 to
to
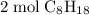
Given the molar mass of the species:
The mass ratio between the two species when the combustion proceeds to completion would thus equal:
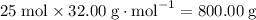 to
to
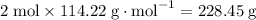
It would thus take
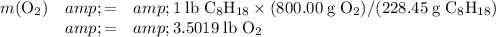
to combust completely one pound of octane.
Apply the mass ratio stated in the question: