Answer: Option (b) is the correct answer.
Step-by-step explanation:
When we increase the temperature of an object then there will be increase in kinetic energy of objects. As a result, collision between the molecules will increase.
Hence, there will be increase in rate of reaction.
Also, K.E =
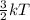
where k = boltzmann constant
T = temperature
So, kinetic energy is proportional to temperature. Hence, when temperature will increase the kinetic energy will also increase.
Thus, we can conclude that an increase in temperature generally affect the rate of a reaction as the rate increases.