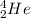₉₂²³³U ⟶ ₉₀²²⁹Th +
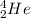
Your equation is:
₉₂²³³U ⟶ ? + α
It becomes easier to balance the equation if we replace the "?" with an element symbol _x^yZ.
Then the equation becomes
₉₂²³³U ⟶ _x^yZ + α
We should also recall that a α particle is helium nucleus. Its nuclear symbol is
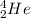 .
.
The equation then becomes
₉₂²³³U ⟶ _x^yZ +
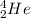
The main point to remember in balancing nuclear equations is that **the sums of the superscripts and the subscripts must be the same on each side of the equation**.
Sum of superscripts: 233 = y + 4, so y =229.
Sum of subscripts: 92 = x + 2, so x = 90.
₉₂²³³U ⟶ ₉₀²²⁹Z +
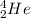
The element with atomic number 90 is thorium, Th.
∴ The nuclear equation is
₉₂²³³U ⟶ ₉₀²²⁹Th +