The correct answer is reaction 2 most likely occurred in their experiment.
So the given two reactions are as follows:
1) Au³⁺(aq) + Co(s) --> Au(s) + Co³⁺(aq)
2) 2Au³⁺(aq) + 3Co(s) --> 2Au(s) + 3Co³⁺(aq)
Mass of Co = 6.087 g
Molar mass of Co = 58.933 g/mol
Moles of Co =
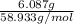 = 0.1032
= 0.1032
Mass of Au = 13.572 g
Molar mass of Au = 196.967 g/mol
Moles of Au =
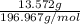 = 0.069
= 0.069
Now based on reaction 1) the molar ratio between Co and Au is 1:1
So as per this reaction 0.1032 moles of Co would produce 0.1032 mole of Au
Again based on reaction 2) the molar ratio between Co and Au is 3:2
So as per this reaction 0.1032 moles of Co would produce
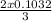 mole of Au
mole of Au
or, As per the second reaction 0.1032 moles of Co would produce 0.0688 moles of Au