Density is equal to the ratio of mass to the volume.
The mathematical expression is given as:
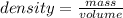
Arrange the above expression in terms of volume, i.e.
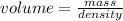
Density of osmium is equal to =
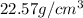
Mass of osmium = 4.80 kg
First, convert the mass in kg to g:
1 kg = 1000 g
Therefore, mass in g =
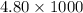 = 4800 g
= 4800 g
Put the values,
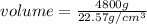
=
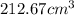 .
.
Hence, volume occupied by osmium =
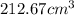 .
.