Step-by-step explanation:
It is known that atomic number is the number of protons present in an atom. Also, when an atom is neutral then number of protons equal number of electrons.
Hence, fluorine has atomic number 9 so there will be 9 electrons in a fluorine atom. It's electronic configuration will be
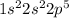 .
.
So, in order to acquire stability fluorine atom will accept one electron from another atom. Therefore, it will result in the formation of
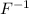 ion.
ion.
Thus, we can conclude that there are total 10 electrons in a fluorine ion.