Number of moles is defined as the ratio of given mass in g to the molar mass.
The mathematical expression is given as:
Number of moles =
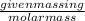
Number of moles of compound = 12.7 moles (given)
As, 1 mole of any compound is equal to
 particles.
particles.
where,
 is Avogadro number.
is Avogadro number.
Formula for calculating particles is given by:
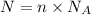
where, N = number of particles, n = number of moles and
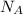 is Avogadro number.
is Avogadro number.
Put the values,
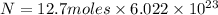
=
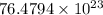 or
or
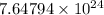
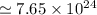
Hence, number of particles of the compound is equal to
