Answer:
The n = 1 shell can contain a maximum of two electrons.
Step-by-step explanation:
An atom is made up of electrons, protons and neutrons. Both protons and neutrons are located in the nucleus of the atom, while electrons are in the shell/s. The shell of an atom is the orbit on which the electrons revolve around the nucleus. The number of shells in an atom extends from the first, n =1, to infinity, n = ∞, depending on the number of electrons in the given atom.
The first shell, n =1, is the ground state and can only contain a maximum of 2 electrons.
Generally, the number of electrons in a shell can be determined by the use of this formula:
2 ×
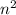
where n is the number of shell required.