Answer:- 3.12 g carbon tetrachloride are needed.
Solution:- The balanced equation is:
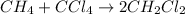
From given actual yield and percent yield we will calculate the theoretical yield that would be further used to calculate the grams of carbon tetrachloride.
percent yield formula is:
percent yield =
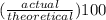
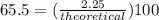
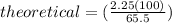
theoretical = 3.44 g
From balanced equation, there is 2:1 mol ratio between dichloethane and carbon tetrachloride.
Molar mass of dichloroethane is 84.93 gram per mol and molar mass of carbon tetrachloride is 153.82 gram per mol.
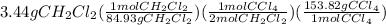
=
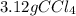
So, 3.12 grams of carbon tetrachloride are needed to be reacted.