Knowing the two variables in this problem are volume (liter) and pressure (atm), we can determine that the law ought to be used would be Boyle's Law.
Boyle's Law: P₁v₁=P₂V₂
Using the question, we can find:
P₁ (initial pressure)=0.24 atm
V₁ (initial volume)=77.0L
P₂ (standard pressure)= 1.00atm
V₂=?
We can rearrange the equation to solve for V₂: V₂=P₁V₁/P₂
Next, we can plug in the values: V₂=
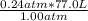
We would receive an answer of 18.48 L. However, it appears that the significant digits would be 3 as both 77.0 and 0.24 have 3 digits. As such, the answer would be 18.5L of gas at 1.00atm.