Answer:- Molecular formula is
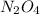 .
.
Solution:- From given percentages We calculate the moles of N and O and then the mol ratio that gives the empirical formula of the compound. Empirical formula mass is calculated for the empirical formula and then we divide the molar mass by empirical formula mass to find out the number of empirical formula units that makes the molecular formula of the compound. empirical formula is multiplied by the number of empirical formula units. the calculations are shown below:
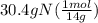 = 2.17 mol N
= 2.17 mol N
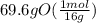 = 4.35 mol O
= 4.35 mol O
Number of moles of N are less than moles of O so we divide the moles of each by 2.17 to get the mol ratio.
N =
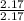 = 1
= 1
O =
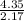 = 2
= 2
The mol ratio of N to O is 1:2 and so the empirical formula is
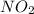 .
.
Empirical formula mass = 14+2(16) = 14+32 = 46
number of empirical formula units =
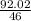 = 2
= 2
So, molecular formula is two times of empirical formula that is
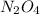 .
.