Answer : 0.026 moles of oxygen are in the lung
Explanation :
We can solve the given question using ideal gas law.
The equation is given below.
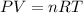
We have been given P = 21.1 kPa
Let us convert pressure from kPa to atm unit.
The conversion factor used here is 1 atm = 101.3 kPa.
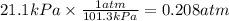
V = 3.0 L
T = 295 K
R = 0.0821 L-atm/mol K
Let us rearrange the equation to solve for n.
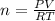
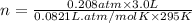
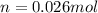
0.026 moles of oxygen are in the lung