The atomic symbol of an element is given as:
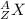
where X is symbol of the element, Z is the atomic number and A is the mass number of the element.
The number of protons present in an atom is the identity of the number of element which is equal to the atomic number of the element, which is unique for every element.
The sum of number of neutrons and protons in an atom is mass number of that atom.
The given information for Atom A is:
34 protons
45 neutrons
34 electrons
Since, number of protons = atomic number. So, atomic number of Atom A is 34. The element with atomic number 34 is selenium,
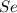 .
.
The number of protons and electrons is same i.e. 34 for an atom thus, the atom A is a neutral atom.
The mass number = number of protons + number of neutrons
Mass number of atom A =
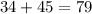
Thus, atom A is
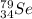 .
.
The given information for Atom B is:
35 protons
45 neutrons
35 electrons
Since, number of protons = atomic number. So, atomic number of Atom B is 35. The element with atomic number 35 is bromine,
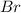 .
.
The mass number = number of protons + number of neutrons
Mass number of atom B =
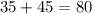
The number of protons and electrons is same i.e. 35 for an atom thus, the atom B is a neutral atom.
Thus, atom B is
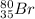 .
.