The formula to determine the average atomic mass is:
Average atomic mass =
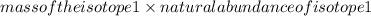 +
+
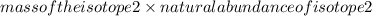 + ........
+ ........
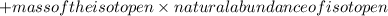 -(1)
-(1)
The atomic mass of first isotope of magnesium = 24 u (given)
The natural abundance of first isotope = 78.70 % (given)
The atomic mass of second isotope of magnesium = 25 u (given)
The natural abundance of first isotope = 10.13 % (given)
The atomic mass of third isotope of magnesium = 26 u (given)
The natural abundance of first isotope = 11.17 % (given)
Substituting the values in formula (1):
atomic weight =

atomic weight =
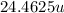
Hence, the atomic weight of naturally occurring isotopic mixture is
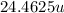 .
.