Conversion factors are defined as the ratio that shows the relationship between two units.
Here, the specific heat = 0.990 J/g
Joule is the unit of energy and g is the unit of mass.
1 Joule = 0.000239006 kilocalorie and 1 g = 1000 mg
Now, 1 J/g is equal to
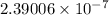
Therefore, 0.990 J/g is equal to (
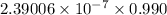 ) kcal/mg
) kcal/mg
=
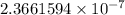 kcal/mg
kcal/mg
Hence, 0.0990 J/g is equal to
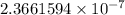 kcal/mg
kcal/mg