Answer:
9.64g/mL
Step-by-step explanation:
Given parameters:
Mass of the metal = 106g
Volume of cylinder = 50mL
Volume difference = 31mL - 20mL = 11mL
Unknown:
Density of the metal = ?
Solution:
To find the density of the metal, we use;
Density =
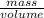
Density =
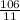 = 9.64g/mL
= 9.64g/mL