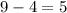Protons are the positively charged particles, electrons are the negatively charged particle and neutrons are neutral particles.
The number of protons is same as the atomic number of element.
The number of electrons is equal to the number of protons as atom consists of same number of protons and electrons.
The number of neutrons is equal to subtraction of number of protons from mass number.
That is, Mass number - Number of protons = Number of neutrons.
a. Bromine (
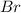 )
)
atomic number of bromine is 35. Thus, number of protons = 35
And, number of electrons = 35
Number of neutrons = Mass number - Number of protons
Mass number of bromine = 79.9
Therefore, Number of neutrons =
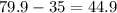 which is approximately equal to 45.
which is approximately equal to 45.
b. Aluminum (
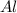 )
)
atomic number of aluminium is 13. Thus, number of protons = 13
And, number of electrons = 13
Number of neutrons = Mass number - Number of protons
Mass number of aluminium = 26.9
Therefore, Number of neutrons =
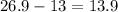 which is approximately equal to 14.
which is approximately equal to 14.
c. Beryllium (
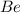 )
)
atomic number of beryllium is 4. Thus, number of protons = 4
And, number of electrons = 4
Number of neutrons = Mass number - Number of protons
Mass number of beryllium = 9
Therefore, Number of neutrons =