Answer :
1. Alloy = Compound
2. Gold = Au
3: Salt Water = Liquid Solution
4. Potassium = K
5. Neutron = No Electric Charge
6. Sugar = Solid Solution
7. Sodium = Na
8. Proton = Positive Electric Charge
Explanation :
1. Alloy : It is a compound that is formed by the combination of one or metals with non-metallic elements. For example : Bronze is a type of alloy that is the combination of tin and copper.
2. Gold : It is chemical element with atomic number 79 and the symbol of gold is (Au). It is yellow, soft, malleable and ductile metal.
3: Salt Water : It is a liquid solution that formed from the combination of salt
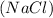 and water
and water
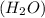 .
.
4. Potassium : It is the chemical element with atomic number 19 and the symbol of potassium is, (K).
5. Neutron : It is a subatomic particle that is present inside the nucleus and it has no charge that means it is has no electric charge.
6. Sugar : It is a solid solution that formed from the combination of sugar and the water.
7. Sodium : It is a chemical element with atomic number 11 and the symbol of sodium is, (Na).
8. Proton : It is a subatomic particle that is present inside the nucleus and it has positive electric charge.