Answer:
Answer is option C (5.0 x 10^22 carbon atoms)
Step-by-step explanation:
To solve this exercise we need to use stoichiometric relations to relate grams of Carbon with atoms of Carbon. These relations are:
a) 1 mol of Carbon atoms = 12 g C (atomic weight of C taken from periodic table)
b) 1 mol of Carbon atomos = 6,022 x 10*23 Carbon atomos (this number is the Avogadros number)
So we will use these two relation to transform grams of Carbon to atoms of Carbon. We will follow this structure:
Unknown variable = Data x "stoichiometric relation 1" x "stoichiometric relation 2"
*Unknown variable = atoms of Carbon
*Data = 1 g of Carbon
*Stoichiometric relation 1 =
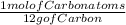
*Stoichiometric relation 2 =
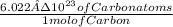
Then we will replace information in general structure:
atoms of C =
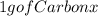
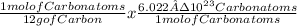
atoms of Carbon = 5,0 x
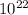 atoms of Carbon
atoms of Carbon