Answer is "n", Principle Quantum Number.
Step-by-step explanation: Bohr's model of Hydrogen atom is based on the quantum mechanics where an assumption was taken that an electron travels in specific orbits around the nucleus.
Energy of the electron in a shell is be given as:
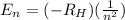
Where,
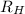 = Rydberg's constant
= Rydberg's constant
n = Integer called as principle quantum number and it corresponds to the different allowed orbits from an electron.
From the equation above we can easily tell that its the Principle Q. No. which determines the electron energy in the hydrogen atom.