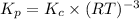Answer : The wrong equation is,
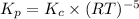
Explanation :
The given equilibrium reaction is,
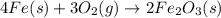
The expression for equilibrium constant in terms of concentration,
![K_c=(1)/([O_2]^3)\\\\K_c=[O_2]^(-3)](https://img.qammunity.org/2019/formulas/chemistry/college/ad3vd4u34eil9wn9wco59l38tbc82lot47.png)
The expression for equilibrium constant in terms of pressure,
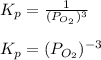
The relation between the equilibrium constant in terms of concentration and equilibrium constant in terms of pressure will be,
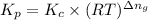
where,
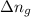 = number of moles of gaseous products - number of moles of gaseous reactants
= number of moles of gaseous products - number of moles of gaseous reactants
R = gas constant
T= temperature
For reaction the given reaction,
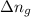 = number of moles of gaseous products - number of moles of gaseous reactants= 0 - 3 = -3
= number of moles of gaseous products - number of moles of gaseous reactants= 0 - 3 = -3
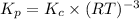
Therefore, the correct equations for equilibrium are,
![K_c=[O_2]^(-3)](https://img.qammunity.org/2019/formulas/chemistry/college/vr8j272k29y01oodplcu1ezemkc8oid40q.png) ,
,
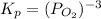 and
and