Answer :
(a) The mass of water produced will be, 22.653 grams.
(b) The mass of NO required will be, 153.09 grams.
(c) The mass of ammonia required will be, 5.8208 grams.
(d) The mass of ammonia required will be, 43.435 grams.
Explanation :
The balanced chemical reaction is,
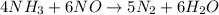
For (a) :
First have to calculate the moles of
 .
.
From the balanced reaction, we conclude that
As, 4 moles of
 react to give 6 moles of
react to give 6 moles of

So, 0.839 moles of
 react to give
react to give
![(6)/(4)* 0.839=1.2585moles of [tex]H_2O](https://img.qammunity.org/2019/formulas/chemistry/middle-school/beqe2e3x2eksgc4saa297dv6upyclsqost.png)
Now we have to calculate the mass of
 .
.
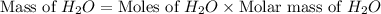
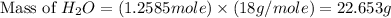
The mass of water produced will be, 22.653 grams.
For (b) :
Now we have to calculate the mass of NO required.
From the balanced reaction, we conclude that
As, 4 moles of
 react to give 6 moles of
react to give 6 moles of
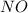
So, 3.402 moles of
 react to give
react to give
![(6)/(4)* 3.402=5.103moles of [tex]NO](https://img.qammunity.org/2019/formulas/chemistry/middle-school/mesvpo34hwnizvr5rspexcjo80wimmqtk0.png)
Now we have to calculate the mass of
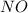 .
.
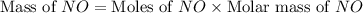
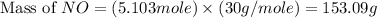
The mass of NO required will be, 153.09 grams.
For (c) :
First we have to calculate the moles of nitrogen gas.
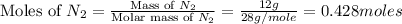
Now we have to calculate the moles of ammonia.
From the balanced reaction, we conclude that
As, 5 moles of
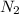 produced from 4 moles of
produced from 4 moles of

So, 0.428 moles of
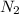 react to give
react to give
![(4)/(5)* 0.428=0.3424moles of [tex]NH_3](https://img.qammunity.org/2019/formulas/chemistry/middle-school/aj3i4w7y32yrv9re62q57kabyy3dnh67dz.png)
Now we have to calculate the mass of
 .
.
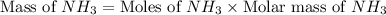
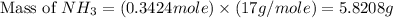
The mass of ammonia required will be, 5.8208 grams.
For (d) :
First we have to calculate the moles of NO.
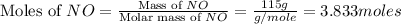
Now we have to calculate the moles of ammonia.
From the balanced reaction, we conclude that
As, 6 moles of
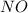 react with 4 moles of
react with 4 moles of

So, 3.833 moles of
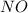 react to give
react to give
![(4)/(6)* 3.833=2.555moles of [tex]NH_3](https://img.qammunity.org/2019/formulas/chemistry/middle-school/z3olsjkd69nzar1rc31jqayum6flknycx3.png)
Now we have to calculate the mass of
 .
.
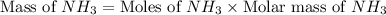
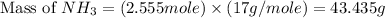
The mass of ammonia required will be, 43.435 grams.